Emission factors
Main article: AP 42 Compilation of Air Pollutant Emission Factors

Beijing air in 2005 after rain (left) and a smoggy day (right)
Air pollutant emission factors are reported representative values that aim to link the quantity of a pollutant released into the ambient air to an activity connected with that pollutant’s release. The weight of the pollutant divided by a unit weight, volume, distance, or time of the activity generating the pollutant is how these factors are commonly stated (e.g., kilogrammes of particulate emitted per tonne of coal burned). These criteria make estimating emissions from diverse sources of pollution easier. Most of the time, these components are just averages of all available data of acceptable quality, and they are thought to be typical of long-term averages.
There are 12 compounds in the list of persistent organic pollutants. Dioxins and furans are two of them and intentionally created by combustion of organics, like open burning of plastics. These compounds are also endocrine disruptors and can mutate the human genes.

E-waste processing in Agbogbloshie, Ghana using open-burning of electronics to access valuable metals like copper. Open burning of plastics is common in many parts of the world without the capacity for processing. Especially without proper protections, heavy metals and other contaminates can seep into the soil, and create water pollution and air pollution.
The United States Environmental Protection Agency has published a compilation of air pollutant emission factors for a wide range of industrial sources.[79] The United Kingdom, Australia, Canada and many other countries have published similar compilations, as well as the European Environment Agency.
Pollutants
Main articles: Pollutant and Greenhouse gas emissions
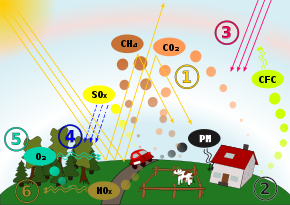
Schematic drawing, causes and effects of air pollution: (1) greenhouse effect, (2) particulate contamination, (3) increased UV radiation, (4) acid rain, (5) increased ground-level ozone concentration, (6) increased levels of nitrogen oxides
An air pollutant is a material in the air that can have adverse effects on humans and the ecosystem.The substance can be solid particles, liquid droplets, or gases. A pollutant can be of natural origin or man-made. Pollutants are classified as primary or secondary. Primary pollutants are usually produced by processes such as ash from a volcanic eruption. Other examples include carbon monoxide gas from motor vehicle exhausts or sulfur dioxide released from factories. Secondary pollutants are not emitted directly. Rather, they form in the air when primary pollutants react or interact. Ground level ozone is a prominent example of a secondary pollutant. Some pollutants may be both primary and secondary: they are both emitted directly and formed from other primary pollutants.
Pollutants emitted into the atmosphere by human activity include:
- Carbon dioxide (CO2): Because of its role as a greenhouse gas it has been described as “the leading pollutant”and “the worst climate pollutant”. Carbon dioxide is a natural component of the atmosphere, essential for plant life and given off by the human respiratory system. This question of terminology has practical effects, for example as determining whether the U.S. Clean Air Act is deemed to regulate CO2 emissions. CO2 currently forms about 410 parts per million (ppm) of earth’s atmosphere, compared to about 280 ppm in pre-industrial times, and billions of metric tons of CO2 are emitted annually by burning of fossil fuels. CO2 increase in earth’s atmosphere has been accelerating.
- Sulfur oxides (SOx): particularly sulfur dioxide, a chemical compound with the formula SO2. SO2 is produced by volcanoes and in various industrial processes. Coal and petroleum often contain sulfur compounds, and their combustion generates sulfur dioxide. Further oxidation of SO2, usually in the presence of a catalyst such as NO2, forms H2SO4, and thus acid rain is formed. This is one of the causes for concern over the environmental impact of the use of these fuels as power sources.
- Nitrogen oxides (NOx): Nitrogen oxides, particularly nitrogen dioxide, are expelled from high temperature combustion, and are also produced during thunderstorms by electric discharge. They can be seen as a brown haze dome above or a plume downwind of cities. Nitrogen dioxide is a chemical compound with the formula NO2. It is one of several nitrogen oxides. One of the most prominent air pollutants, this reddish-brown toxic gas has a characteristic sharp, biting odor.
- Carbon monoxide (CO): CO is a colorless, odorless, toxic gas. It is a product of combustion of fuel such as natural gas, coal or wood. Vehicular exhaust contributes to the majority of carbon monoxide let into the atmosphere. It creates a smog type formation in the air that has been linked to many lung diseases and disruptions to the natural environment and animals.
- Volatile organic compounds (VOC): VOCs are a well-known outdoor air pollutant. They are categorized as either methane (CH4) or non-methane (NMVOCs). Methane is an extremely efficient greenhouse gas which contributes to enhanced global warming. Other hydrocarbon VOCs are also significant greenhouse gases because of their role in creating ozone and prolonging the life of methane in the atmosphere. This effect varies depending on local air quality. The aromatic NMVOCs benzene, toluene and xylene are suspected carcinogens and may lead to leukemia with prolonged exposure. 1,3-butadiene is another dangerous compound often associated with industrial use.
- Particulate matter/particles, also known as particulate matter (PM), atmospheric particulate matter (APM), or fine particles, are microscopic solid or liquid particles suspended in a gas. Aerosol, on the other hand, is a mixture of particles and gas. Volcanoes, dust storms, forest and grassland fires, living plants, and sea spray are all sources of particles. Aerosols are produced by human activities such as the combustion of fossil fuels in automobiles, power plants, and numerous industrial processes. Averaged worldwide, anthropogenic aerosols – those made by human activities – currently account for approximately 10% of our atmosphere. Increased levels of fine particles in the air are linked to health hazards such as heart disease, altered lung function and lung cancer. Particulates are related to respiratory infections and can be particularly harmful to those with conditions like asthma.
- Persistent free radicals connected to airborne fine particles are linked to cardiopulmonary disease.
- Toxic metals, such as lead and mercury, especially their compounds.
- Chlorofluorocarbons (CFCs): Emitted from goods that are now prohibited from use; harmful to the ozone layer. These are gases emitted by air conditioners, freezers, aerosol sprays, and other similar devices. CFCs reach the stratosphere after being released into the atmosphere. They interact with other gases here, causing harm to the ozone layer. UV rays are able to reach the earth’s surface as a result of this. This can result in skin cancer, eye problems, and even plant damage.
- Ammonia: Emitted mainly by agricultural waste. Ammonia is a compound with the formula NH3. It is normally encountered as a gas with a characteristic pungent odor. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to foodstuffs and fertilizers. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceuticals. Although in wide use, ammonia is both caustic and hazardous. In the atmosphere, ammonia reacts with oxides of nitrogen and sulfur to form secondary particles.

